
Hectorol
Injection Prescribing Information
Patients
For U.S. Healthcare Professionals Only
About Hectorol® (doxercalciferol) injection
Mechanism of Action
-
Hectorol®:
a prodrug1,2,3Prodrugs are compounds that require chemical conversion by metabolic processes before they become an active pharmacological agent.4 Hectorol is a synthetic vitamin D2 analog that gets converted to a biologically active form of vitamin D2 in vivo, without the involvement of the kidneys, to help regulate iPTH serum levels in patients with CKD.5 Doxercalciferol, the active ingredient in Hectorol, is inert until activated by the liver, making it a prodrug.2,3
The consequence of in vivo activation of Hectorol is the release of the major metabolite of doxercalciferol (1α,25-[OH]2D2) with a mean elimination half-life of approximately 32 to 37 hours.
How is active
vitamin D formed?
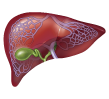
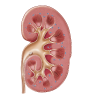
1. Person without CKD
Inactive vitamin D comes from dietary sources and from the exposure of skin to sunlight. It is activated by the liver and kidneys to form active vitamin D.9
 +
+
 +
+
 =
Active
=
Active
vitamin D
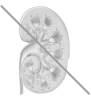
2. Person with CKD
Inactive vitamin D provided by Hectorol is activated by the liver, without the need for activation by the kidneys, to form active vitamin D.9
 +
+
 +
+
 =
Active
=
Active
vitamin D
